
amtiskaw
-
Posts
573 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Plant Articles
Fish Articles & Guides
Clubs
Gallery
Posts posted by amtiskaw
-
-
maybe the final product is the "special ingredient" that all the fancy waters seem to have tough? would explain the crystal blue springs too :slfg:
LOL, you'd end up with fish looking like your avatar - tweaker tropicals! :nilly:
-

direct syphon into a "fancy water for rich people" truck ?
Haha, don't think I'll try drinking methylamine any time soon :dead%fish
-
http://www.nzherald.co.nz/nz/news/artic ... d=10852018
Is there some sort of black market for fish in NZ?
-
I like the new layout, although it seems mostly cosmetic with some new privacy features.
I just clicked on Library and there were all my pics, in the same album structure as before the change.
I can't see what the fuss is about :dunno:
-
Just read that Dave Grohl will be back on the drums for the Queens of the Stone Age on next year's album

-
little fact to blow your mind... Glass is technically a Liquid.
Bzzzt - Glass is an amorphous solid. It's an urban myth that it's a liquid that slowly flows over time :smln:
-
I saw QotSA a few years ago at the BDO. The PA kept cutting out and spoilt their set, but they did another set later on on a different stage, which I was lucky enough to see. One of the best live rock acts ever IMO :bounce:
-
Good music is not a fact, it's an opinion.
+1 There's no point in arguing about something as subjective as music. Although I feel sorry for people who say there's been no good music since or that genre x is crap and I only listen to genre y.
Try expanding your horizons a little, and use some interweb services to find some new bands for you. There's plenty of sites that'll let you put in some favs and then suggest something new for you.
The interweb is an awesome source for music :bounce:
-
Do yourself a favour - get an external USB drive and get in the habit of backing your data up every week :sage:
Use a robocopy script to do a weekly differential backup 8)
-
I did some more reading on this. Apparently it is legit, and the plan is to use renewable energy sources (solar, hydro) to supply energy for the process.
Pretty cool if it is commercially viable 8)
-
Unexplained disappearance of scammers in 3.....2.....1....
There, fixed that for you
-
Haha, it's the
bird :slfg: -
How do you know they're staged shots?
Becoming quite common now days, not 100% sure how it's achieved but I know they are set up shots. Not sure if the bugs are dead or glued but I cant imagine them sticking around after having all that water sprayed on them.http://500px.com/photo/8196182
This guy has been doing it for a while and has been in National Geographic a few times.
-
I doubt we'll get to the bottom of this...
-
-
Cold As Ice - Foreigner
-
Nero - BBC1 Essential Mix recorded live at Hackney Weekend, 24th June 2012
Play it loud with the bass up :bounce:
-
Bubbles displace water as they form, but they're basically at the same pressure as the air outside the water is what I'm saying. If they were formed in deep water they'd be at a higher pressure than the same volume of air at the surface, but the change in pressure in a few feet of water is negligible.
-
its not but when the O2 content is higher (and CO2 lower) it will transfer a lot quicker, i.e. if the air was very high in CO2 then CO2 is going to be absorbed until the concentrations are more equal and virtually no O2 would be absorbed
Agreed, but you've lost me again - I don't see what that has to do with what we're discussing? The air pumping through your airstone is the same as the air at the surface of the tank.
I'd imagine the surface area of all the bubbles combined greatly exceeds the area of the tank's surface, therefore the gas exchange of a bubbler exceeds the gas exchange at the tank's surface surface, and the benefit is direct gas exchange by the bubbles, not just increased surface agitation.
Maybe someone can google a reference on the rate of oxygen dissolution in a pressurized air bubble but I suspect it's not very quick. If you look at a planted tank you see lots of bubbles of pure oxygen being formed, and they seem to stick around for quite a while.Vaguely recalling high school physics, I'd have thought you'd get more gas dissolving into water if bubbles are at a higher pressure than ambient atmospheric pressure? But then I don't think bubbles would be at a higher pressure in just a meter or so of water anyhow.
BTW, sorry to the OP for the hijack :oops:
-
gas exchange does occur within the bubbles, but the surface of the tank far exceeds the bubbles for the gas exchange due to the fact the air is also constantly refreshing itself, that is why you get almost the same amount of as exchange from a powerhead breaking the surface than as from an airstone.
Any chance you can link to some research that proves this? I don't get your refreshing comment, sorry. I wouldn't have thought each bubble's going to exhaust it's O2 exchanging capacity in the few seconds it's submersed?
-
Oxygenation occurs at the surface of the water which is why the surface area of the water is used to calculate the carrying capacity of the tank.
The air bubblers just breaks up the water surface which increases the oxygen exchange at the surface.
I disagree. The surface area of the bubbles would far exceed the area of the tank's surface, so gas exchange is increased directly.
-
The main one - it's part num 734315 in this pic
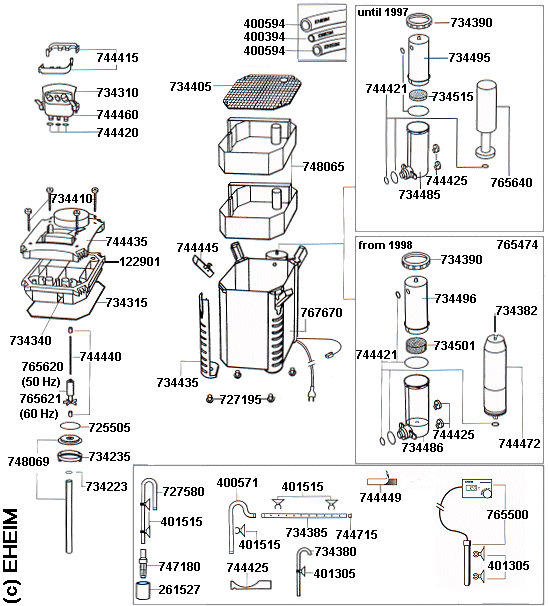
It's part num 734315 in this pic. Is this the same as your filter?
-
Try lubing the seal with Vaseline. Remove it completely, clean and dry it, then lube it before putting it back in.
-
@ Amtiskaw - where are the fish?
Still on Earth at the moment. They're catching the next rocket up. Did you follow the link in my post above? It'll be really interesting to see how they adapt to micro-gravity in orbit :dead%fish :dead%fish :dead%fish :dead%fish :dead%fish
I bet that's the most expensive aquarium ever, in terms of $$ per ml


Parrot With Own Mobility Scooter
in The Off Topic Fishroom
Posted